Recently, the use of CAR-T cells has made a major impact on the therapy for a subset of hematologic malignancies including non-Hodgkin lymphoma (NHL) and multiple myeloma. In relapsed/refractory NHL where historically most patients did not respond to chemotherapy, CAR-T cell therapy can lead to initial remission rates >50%. Despite promising outcomes, there are major unmet needs to develop rapid, cost-effective, and scalable CAR-T manufacturing platforms that lead to efficacious and affordable autologous CAR-T cell products. Major barriers to widespread access to CAR-T cell therapy include weeks to months long delays from time of prescription to infusion that leads to disease progression in a significant subset of patients, very elevated costs that preclude access for a majority of patients world-wide, and high relapse rates (~estimated 30-60% within one year) in patients who initially achieve remission.
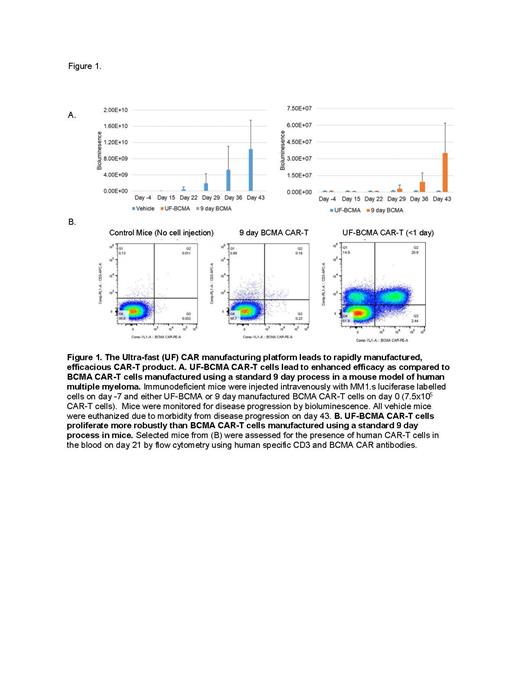
To help address these barriers, we developed an ultra-fast CAR-T manufacturing platform (UF-CAR) that allows production of highly cost-effective CAR-T product in less than 24 hours. The manufacturing workflow was specifically designed to be simple, low cost and rapid to enable its formulation in healthcare facilities without sophisticated equipment or personnel expertise. In addition, we hypothesized that this manufacturing workflow would generate a product that demonstrates dramatically improved efficacy and CAR-T expansion in vivo. Using a BCMA CAR we manufactured CAR-T cell product using both our ultra-fast manufacturing workflow and a traditional 9-day CAR-T cell manufacture workflow. Both workflows led to nearly identical levels of CAR surface expression on the T cells (67% and 66% CAR expression on the UF-BCMA product and 9-day product respectively). To compare the activity of the products, we injected immunodeficient mice intravenously with luciferase labelled MM1.s cells followed by a single intravenous injection of CAR-T cells on day 0. As seen in figure 1A, both products demonstrated efficacy, but the UF-BCMA product induced durable disease control while disease progression started to occur with the 9-day product on day 29 after CAR-T injection. Consistent with the improved efficacy, circulating levels of the UF-BCMA CAR-T cells in the mouse blood were found to be up to 100x higher than the levels of the 9-day BCMA CAR-T product (Figure 1B). Similar increased potency and marked increases in circulating CAR-T cells were observed in a mouse model of human lymphoma when comparing a CD19 CAR manufactured using the UF-CAR workflow as compared to a standard CAR-T manufacturing process. To assess the safety and clinical potential of CAR-T products manufactured with the UF-CAR platform, a phase 1 trial was recently initiated using a CD19 CAR-T product (UF-Kure19). This trial that is open to patients with relapsed/refractory NHL completed treatment of its first patient who had relapsed mantle cell lymphoma. UF-Kure19 product was manufactured successfully in less than 1 day and demonstrated CAR expression of 41%. The product passed all release criteria for infusion and was administered to the patient after lymphodepletion with fludarabine 30mg/m2 and cyclophosphamide 500mg/m2 daily for 3 days. UF-Kure19 cells expanded robustly in the patient and peaked around day 10 (23% of circulating CD3) with a strong skewing of CD4/CD8 cells (93% CD8+ T cells on day 10). The patient was found to be in complete metabolic remission by day 28 and ongoing remission was confirmed on day 60. We have developed an ultra-fast CAR-T cell manufacturing platform that enables low-cost production in less than one day. In vitro and animal data suggest this fast-manufacturing platform leads to potent CAR-T cells. Early human testing indicates CAR-T cells generated using this novel system have clinical activity against hematologic malignancies.
Disclosures
Stadel:Kure.ai: Ended employment in the past 24 months; immunomic therapeutics: Current Employment. Idippily:Kure.ai: Current Employment. Giraudo:Kure.ai: Current Employment. Van Besien:Orca: Research Funding; Avertix: Current equity holder in private company; Calibr: Research Funding; BMS: Research Funding; Actinium: Research Funding; Moprhosys: Consultancy; SNIPR: Consultancy; Precision Biosciences: Research Funding; Intellia: Consultancy; Hemogenyx: Consultancy, Current equity holder in publicly-traded company; Incyte: Consultancy. Wu:Promab: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Deng:Janssen: Ended employment in the past 24 months. Wald:Kure.AI: Consultancy, Current equity holder in private company, Patents & Royalties; Kure.ai Therapeutics: Consultancy, Current equity holder in private company; CuronBiotech: Consultancy, Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal